PERMEOS Electro-Chemotherapy System by Vitalis Technologies (Formerly by Tenon Meditech), India, is fast emerging as a powerful tool in management of cutaneous and sub-cutaneous cancer tumours.
Advanced stage head and neck cancer tumours and cutaneous and sub-cutaneous metastatic tumours invariably pose a major ‘treatment challenge’. They are often unresponsive to regular treatment modalities of chemotherapy or radiation therapy. Surgery is invariably either not feasible, or difficult, due to their size and location. Overall health condition of the patient can also hinder treatment options. Left with no treatment modalities, such patients would earlier have only one recourse, i.e., to endure.
Electro-chemotherapy (ECT) is a new and advanced globally proven tool for management of such tumours. ECT marks a paradigm shift in tumour treatment by offering an effective solution for ‘such difficult to treat tumours’. PERMEOS Tumour Management system is a diligently tested and clinically proven electro-chemotherapy system. Government of India recognises it as a socially important treatment modality.
Product development of PERMEOS was supported by

Nidhi Prayas grant from the Government of India.

Development of smart applicators for electro-chemotherapy


POWERFUL
IMPACT
- 30 minutes procedure
- Pain relief within 48 Hours
- Notable tumour regression in 2 to 4 weeks
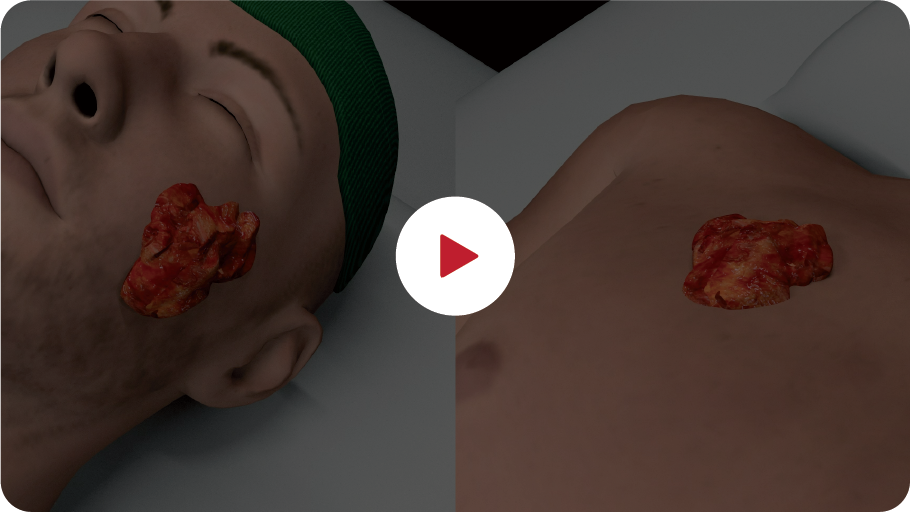
Drag left/right for full view
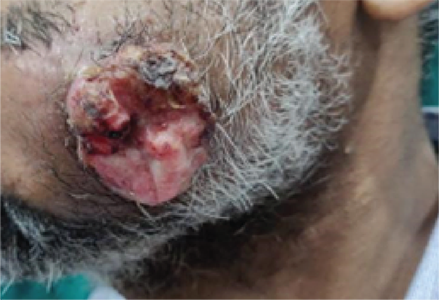
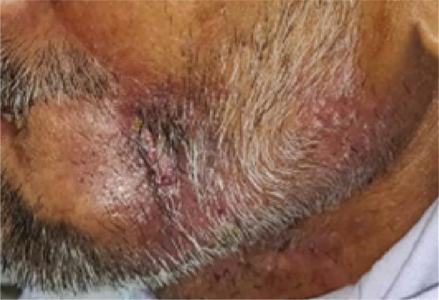
Patient treated with two sessions of PERMEOS electro-chemotherapy
Treatment Impact
Advanced stage head and neck cancer tumours and various types of metastatic tumours pose ‘just’ as big a challenge to the patient, as they do to the doctor. Large and painful, they leak blood and secretions. Their size and pain can hinder functions like eating, swallowing, speaking and breathing. They affect the patient’s quality of life adversely.
Permeos electro-chemotherapy (ECT) of such tumours can achieve the much needed relief through:
- Regression & healing of tumour (total/partial)
- Tumour pain control
- Control of tumour bleeding & secretions.
Control of pain, bleeding and secretions can start within 48 hours of an electro-chemotherapy session. 2 to 4 weeks are needed to see notable tumour regression. Treatment can be repeated if necessary.
No/minimal side-effects
Side-effects of ECT treatment are nil/minimal as quantity of chemotherapeutic drug used is very small.
Procedure takes just 30 minutes
Permeos electro-chemotherapy is a local tumour treatment. If done under local anaesthesia, which is usually the case, it requires only 30 minutes. Patient can be discharged after a short observation period.
Types Of Tumours
- Head & neck squamous cell carcinoma
- Salivary gland hypernephroma
- Adenocarcinoma of the salivary glands
- Kaposi sarcoma
- Transitional cell carcinoma
- Basal cell carcinoma
- Malignant melanoma
- Adenocarcinoma of the breast
Palliative, adjuvant & neoadjuvant treatment
Electro-chemotherapy has a very important application as a palliative tool in irresectable cutaneous and sub-cutaneous tumours non-responsive to regular chemotherapy and radiation therapy.
Electro-chemotherapy can be applied concurrently with other treatment modalities. As a neoadjuvant treatment, it is used prior to surgery to facilitate resection of tumours which are otherwise irresectable or difficult to operate due to their size/location. It is used adjuvantly along with chemotherapy and/or radiation therapy for better tumour specific results. It can be applied to earlier irradiated tumours and tumours with fungating mass.
DEVICE
HIGHLIGHTS
- Diligently tested
- Clinically proven
- Novel safety features


PERMEOS Tumour Management System has been designed in consultation with doctors and other stake holders. PERMEOS has integrated several safety and convenience features for users and patients. It is diligently tested to ensure high safety standards. It has an easy to use interface and easy portability for movement between locations.

Novel safety features
- • Integrated current and voltage control
- • Phase reversal protection
- • Locking system to secure the electrodes firmly

Clinically proven
Permeos is clinically proven. It is being used in cancer hospitals in India.

Other novel features
- • Integrated software app for capture and storage of patient data and tumour images (image capture by camera)
- • Voltage check to ensure treatment efficay
- • Long electrodes for better reach in oral cancers
- • Printable device generated report.

Testing
Safety and efficacy tested in government approved facilities at
- IIT-Bombay, Mumbai
- National Toxicology Centre, Pune
- National Electrical & Electronic Laboratory (West), Pune